SOLVED:Palladium crystallizes with a face-centered cubic structure. It has a density of 12.0 g/cm3, a radius of 138 pm, and a molar mass of 106.42 g/mol. Use these data to calculate Avogadro's

Document - Problem#1 Palladium crystallizes in a face-centered cubic unit cell Its density is 12.023 g\/cm3 Calculate the atomic radius of palladium | Course Hero

OneClass: Palladium crystallizes with a face-centered cubic structure. It hasa density of 12.0 g/cm3,...
![Platinum crystallize in a face centered cube crystal with a unit cell length of 3.9231 Å. The density and atomic radius of platinum are respectively: [Atomic mass of Pt = 195] Platinum crystallize in a face centered cube crystal with a unit cell length of 3.9231 Å. The density and atomic radius of platinum are respectively: [Atomic mass of Pt = 195]](https://d10lpgp6xz60nq.cloudfront.net/ss/web/422402.jpg)
Platinum crystallize in a face centered cube crystal with a unit cell length of 3.9231 Å. The density and atomic radius of platinum are respectively: [Atomic mass of Pt = 195]
If the radius of palladium is 248 pm and the lattice type is body centered cubic, what is the - Sarthaks eConnect | Largest Online Education Community

SOLVED:Palladium crystallizes with a face-centered cubic structure. It has a density of 12.0 g/cm3, a radius of 138 pm, and a molar mass of 106.42 g/mol. Use these data to calculate Avogadro's

1. The density of solid Ni is 8.90 g/cm^3. How many atoms are present per cubic centimeter of Ni? 2. As a solid, Ni adopts a face-centered cubic unit cell. How many

OneClass: Palladium crystallizes with a face-centered cubic structure. It hasa density of 12.0 g/cm3,...

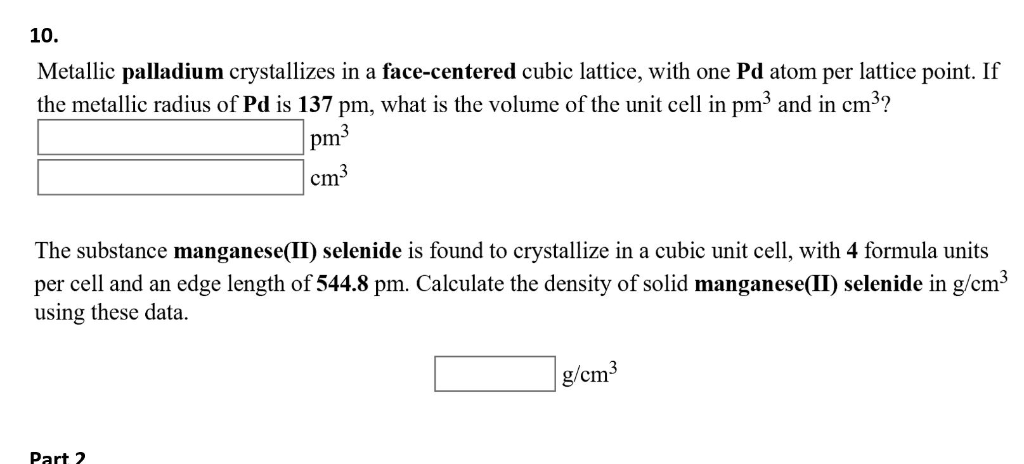
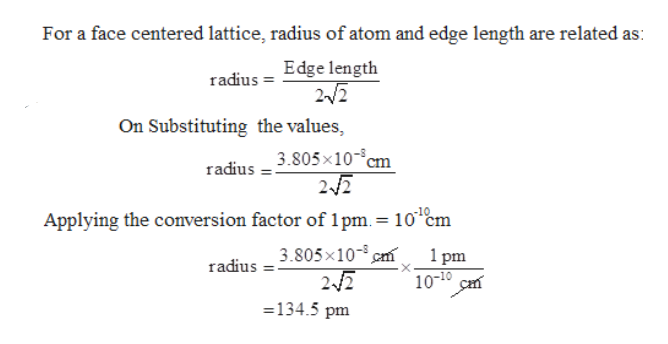
Document - Problem#1 Palladium crystallizes in a face-centered cubic unit cell Its density is 12.023 g\/cm3 Calculate the atomic radius of palladium | Course Hero

Chapter 3 Homework - Chapter 3 Homework Textbook Problems 9 17 22 25 33 40 44 46 71 79 Additional problems and solutions Problem#1 Palladium | Course Hero

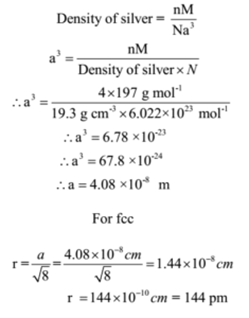
Gold (atomic mass = 197 u) has atomic radius = 0.144 nm. It crystallises in face centred unit cell. Calculate the density of gold. (No = 6.022xx10^(23)mol^(-1))
roblem.docx - problem#1 Palladium crystallizes in a face-centered cubic unit cell Its density is 12.023 g\/cm3 Calculate the atomic radius of palladium | Course Hero

SOLVED:Palladium crystallizes with a face-centered cubic structure. It has a density of 12.0 g/cm3, a radius of 138 pm, and a molar mass of 106.42 g/mol. Use these data to calculate Avogadro's